![]() Children are absolutely essential to the success of COVID-19 vaccination efforts, as children aged 0-15 years compose nearly 20% of the United States total population. However, the distribution of children is highly uneven across both geographic areas and by racial/ethnic populations in the U.S. As a result, some communities have a high proportion of their total population that is currently age-ineligible to receive the vaccine. Public health goals to achieve long-term herd immunity to COVID-19 are dependent on the availability and acceptability of safe vaccines for children of all ages.
Children are absolutely essential to the success of COVID-19 vaccination efforts, as children aged 0-15 years compose nearly 20% of the United States total population. However, the distribution of children is highly uneven across both geographic areas and by racial/ethnic populations in the U.S. As a result, some communities have a high proportion of their total population that is currently age-ineligible to receive the vaccine. Public health goals to achieve long-term herd immunity to COVID-19 are dependent on the availability and acceptability of safe vaccines for children of all ages.
Child vaccine progress and eligibility
There are currently 89 COVID-19 vaccines being tested in clinical trials on humans. While pharmaceutical companies are making progress with pediatric trials, children 15 years and younger are still ineligible to receive the three vaccines approved by the U.S. Food & Drug Administration (FDA) for Emergency Use Authorization. Pfizer-BioNTech and Moderna recently began clinical trials in infants and children aged 6 months to 11 years, and both companies have had vaccine trials underway in adolescents aged 12-18 years since late 2020.
Pfizer-BioNTech issued a press release on March 31, 2021, announcing their vaccine safely protects adolescents aged 12-15 years. The FDA is currently reviewing data submitted by Pfizer-BioNTech in order to expand approval of the vaccine. While adolescents aged 12-15 years are likely to be eligible for vaccination this summer, younger kids will likely not be eligible until early 2022. Determining safe and optimal dosages for infants and elementary school children is a key metric.
Johnson & Johnson has also announced plans to expand vaccine trials in various age groups, including infants. Most recently, after six women of reproductive age developed a rare disorder involving blood clots, the Centers for Disease Control and Prevention (CDC) and FDA paused vaccine use from April 13-23, 2021. The FDA Emergency Use Authorization was never revoked, and their pediatric trials are still recruiting (see Table 1 for complete pediatric trial information).
Overall, the ongoing enrollment of children into several vaccine trials is good news. It’s also significant that adolescents will have the opportunity to be vaccinated within the next six months if the Pfizer-BioNTech request is approved by the FDA. In Florida, for example, adolescents aged 12-17 years comprise 48% of all pediatric COVID-19 cases.
Pfizer-BioNTech and Moderna—both mRNA vaccines—have demonstrated greater than 90% efficacy against symptomatic COVID-19 illness in adults, and evidence is mounting that they may also protect vaccinees against asymptomatic infection. This finding could be particularly important if it also holds true for children, among whom asymptomatic infections are common. Preliminary evidence for the Pfizer-BioNTech vaccine also suggests that after one shot, viral loads may be lowered even when breakthrough infections occur. Reduction in viral loads has implications for reduced shedding, and hence, transmission. Nevertheless, it will take time to determine the extent to which these correlates of protection yield a significant reduction in viral transmission.
Most scientists agree that COVID-19 will unlikely be eradicated given widespread persistent outbreaks, new variants, and challenges to vaccine uptake. Instead, COVID-19 will more likely become endemic in many populations. And we’re all familiar with the endemic rhinovirus—the common cold. The cycle often goes that children are infected with the virus, develop symptoms, then spread the virus to adults in whom immunity has waned over time. Thus, vaccinating children against COVID-19 will surely help in breaking chains of viral transmission.
Herd protection and the vaccine age-ineligible population
The percentage of the total U.S. population currently age-ineligible for the COVID-19 vaccine—that is, children aged 0-15 years—varies widely both geographically and by race/ethnicity.
The COVKID Project has a vaccine age-ineligible dashboard that demonstrates this variation through the use of several maps. As demonstrated in the following figure, Appalachia, New England, and Florida are regions with smaller pediatric populations, and thus, will benefit most from adult vaccination campaigns. In contrast, the Mississippi Delta, Utah, Arctic Alaska, Rio Grande Valley, and West Texas have pediatric populations greater than 24%. On a state-based level, the vaccine age-ineligible population ranges from 15.8% in Vermont to 25.7% in Utah.
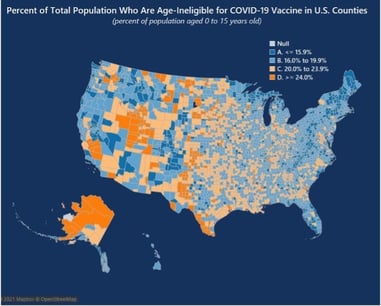 Figure 1: The COVKID Project, Vaccine Age-Ineligible Population Dashboard
Figure 1: The COVKID Project, Vaccine Age-Ineligible Population Dashboard
Additionally, our research has shown that Latino, Black, American Indian, and Alaska Native populations have the highest percentages of persons who are age-ineligible for the vaccine. Worse yet, these are the same communities that suffer the highest burden of COVID-19 incidence and mortality.
Efforts addressing community-level access to the vaccine and vaccine hesitancy have renewed urgency. The Pew Charitable Trusts reports that Arizona, Vermont, and Washington are using a vulnerability index that includes census data on poverty, lack of transportation, and crowded housing to identify vulnerable populations and tailor public health messages regarding vaccination. New Jersey is using a vulnerability index to help decide where to place COVID-19 vaccination clinics. And Arizona, Utah, Missouri, and North Carolina have prioritized Black, Latino, and Native American residents in their vaccine distribution plans.
The Kaiser Family Foundation COVID-19 Vaccine Monitor provides ongoing tracking of the public’s attitudes towards COVID-19 vaccination. The most recent survey reported increased interest and uptake across all racial and ethnic groups.
Conclusion
Critical components for successful vaccination of pediatric populations include successful completion of child clinical trials with tolerable safety and biomarker results; approval for Emergency Use Authorization after completion and review of vaccine safety trials; and engagement with family medical decision makers (e.g., parents, grandparents, guardians) about getting kids vaccinated.
In the meantime, we must persist with proper masking, physical distancing, and public health efforts to vaccinate all currently eligible populations.
Table 1: Current and Planned COVID-19 Vaccination Trials Involving Pregnant Women & Children
|
Trial |
Age |
Doses |
Study Phase and Endpoints |
Estimated Completion Date |
|
Pfizer-BioNTech (NCT04713553) |
12-15 years |
20mcg 30mcg 2 dose schedule 21 days apart |
Phase 3 – evaluating safety, tolerability, and immunogenicity of SARS CoV-2 vaccine in healthy individuals |
5/11/2021
|
|
Moderna “TeenCOVE” |
12-17 years |
100mcg 2 dose schedule 28 days apart |
Phase 2/3 – evaluating safety, reactogenicity, and effectiveness in healthy adolescents |
6/20/2022 |
|
6 months - 11 years |
2 dose schedule 21 days apart |
Phase 1/2/3 – evaluating safety, tolerability, and immunogenicity in healthy children (vaccine effectiveness will be inferred through immuno-bridging to the 16-25 year old population Phase 3 trial) |
3/1/2022 |
|
|
Moderna “KIdCOVE” |
6 months - 11 years |
28 days apart |
Phase 2/3 – evaluating safety, tolerability, reactogenicity, and effectiveness in healthy children |
6/10/2023 |
|
Pfizer-BioNTech (NCT04754594) |
Pregnant persons aged 18+ years & 24-34 weeks gestation |
Phase 2/3 – evaluating safety, tolerability, and immunogenicity of vaccine in healthy pregnant women |
6/27/2022 |
|
|
Johnson & Johnson |
12-17 years |
1 & 2 dose schedules |
Phase 2a – evaluating 2 dose schedules in healthy adolescents |
12/15/2021 |
**Feature photo obtained with standard license on Shutterstock.
Interested in other articles like this? Subscribe to our bi-weekly newsletter
Interested in contributing to the Harvard Primary Care Blog? Review our submission guidelines

Rebecca B. Garcia, MSN, FNP-C, is a family nurse practitioner and certified diabetes educator. She is Director of Regional Clinical Operations at Premise Health and a Healthcare Fellow with the Women’s Institute for Independent Social Enquiry. Her professional interests focus on social determinants of health, syndemics, COVID-19 return to work safety, decoding racial bias in practice, and lifestyle medicine.
 Janelle M. Menard, PhD, is a medical anthropologist and social epidemiologist with expertise in mixed methods and community‐based participatory research. She advocates for an integrative biocultural and socioecological research framework in health disparities research, and her fieldwork in the French Caribbean and among Latin American and Caribbean immigrants in the U.S. has informed interventions to improve health equity. She is currently Director of Education for The COVKID Project and Vice President of the Women's Institute for Independent Social Enquiry.
Janelle M. Menard, PhD, is a medical anthropologist and social epidemiologist with expertise in mixed methods and community‐based participatory research. She advocates for an integrative biocultural and socioecological research framework in health disparities research, and her fieldwork in the French Caribbean and among Latin American and Caribbean immigrants in the U.S. has informed interventions to improve health equity. She is currently Director of Education for The COVKID Project and Vice President of the Women's Institute for Independent Social Enquiry.
 Elizabeth B. Pathak, PhD, MSPH, is an epidemiologist who has spent 30 years conducting research on geographic, socioeconomic, and racial disparities in health. She is a passionate advocate for methodologically rigorous scholarship which seeks to uncover the power relations that perpetuate socioeconomic and health inequities. She is Director of The COVKID Project and President of the Women’s Institute for Independent Social Enquiry.
Elizabeth B. Pathak, PhD, MSPH, is an epidemiologist who has spent 30 years conducting research on geographic, socioeconomic, and racial disparities in health. She is a passionate advocate for methodologically rigorous scholarship which seeks to uncover the power relations that perpetuate socioeconomic and health inequities. She is Director of The COVKID Project and President of the Women’s Institute for Independent Social Enquiry.
- Share
-
Permalink